Which of the Following Acids Ionizes Only Partially in Water
The spectator ions in the reaction between aqueous. For sulfuric acid H2SO4 only the first hydrogen ionizes completely since its a diprotic acid.

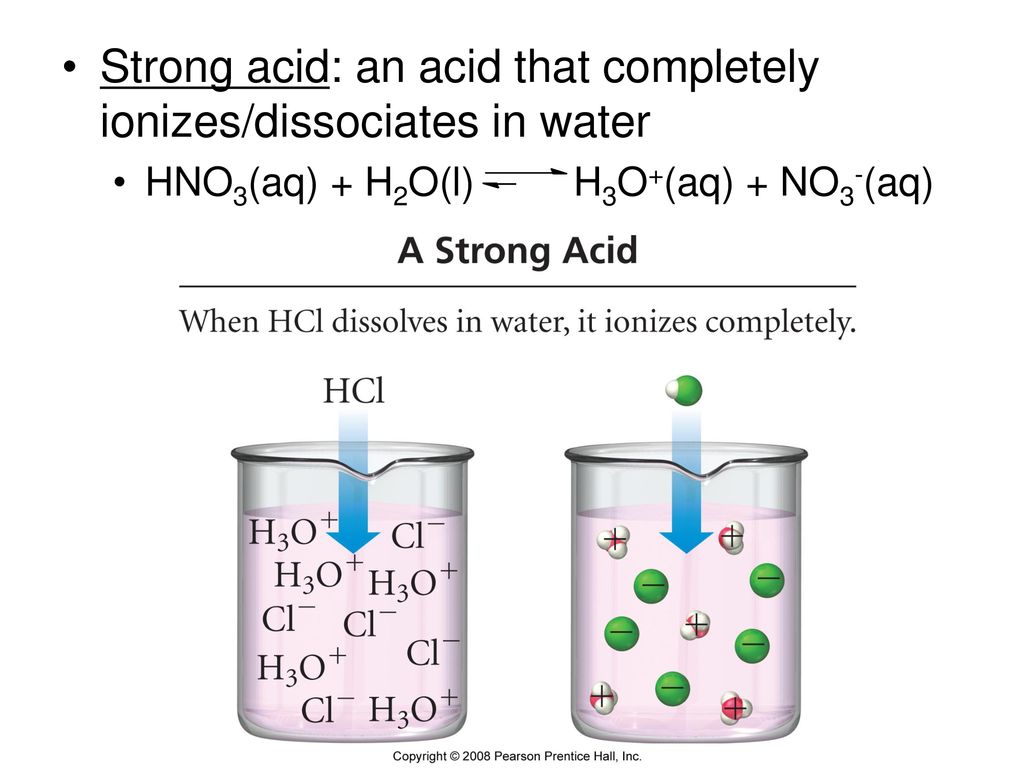
Acids And Bases Topics To Be Covered Definitions Of Acids And Bases Ppt Download
They are partially ionized in water.
. Weak acid like acetic acid partially ionizes in water as their structure is complex. Wiki User 2008. An acid produces hydronium ions in water.
In an aqueous solution the H ion binds to a water molecule A water molecule that takes on a hydrogen ion is called a hydronium ion H3O. Which one of the following statements regarding a weak acid is NOT correct. A weak acid neutralizes bases.
HCN H2O What solute particles are present in an. An acid ionizes in water. In aqueous solution phosphoric acid behaves as a triprotic acid having three.
A strong acid will completely ionize in water while a weak acid will only partially ionize. It is known that acetic acid is a weak acid. Hydrogen gas is produced.
Pure anhydrous phosphoric acid is a white solid which melts at 4235 C to form a viscous liquid. Acidis any substance that ionizes partially or completely in water to give hydrogen ions which associate with the solvent to give hydronium ions H 3. CH 3 COOH is a weak acid because it ionizes only partially in aqueous solution to form H and CH 3 COO ions.
When an acid reacts with an active metal a. This preview shows page 14 - 16 out of 16 pages. What do weak bases do in water.
The dissociation constant is a function of pH. A weak acid ionizes in water to produce hydronium ions. 3An electrolyte is a substance that dissolves in water to produce a solution that conducts electricity.
A weak acid is an acid that ionizes only slightly in an aqueous solution. Acids ionize only partially in water. HCN aq H 2 O l H 3 O aq CN - aq K a 501 x 10 -10 a What assumption can be made when calculating the pH of this solution.
HCl is an acid according to the arrhenius definition because when placed in water it ionizes completely to form H ions and Cl- ions. B Nitric acid is about 100 times more acidic than acetic acid because nitric acid solution contains about 100 times the number of hydronium ions. Acid consisting of hydrogen oxygen and a third element oxyacid from the latin word acidus meaning sour acid composed of hydrogen and one other element binary acid completely ionizes in water strong acid donates one proton per molecule of acid monoprotic acid one of the products of an acid reacting with a base water partially ionized acid.
Hydrogen cyanide is a weak acid forming medium soluble gas the solution is called hydrocyanic acid in water. The number of atoms. H3PO4 H H2PO4- Ka1 75.
For a list of the strong acids and bases try this. A substance present in smaller quantity is known as solute and a substance present in larger quantity is known as solvent. A strong acid is an acid which is completely ionized in an aqueous solution.
Which of the following is a hydroxide ion. Since there are different degrees of ionization there are different levels of weakness. CH 3 COOH CH 3 COO H.
The degree to which a weak acid ionizes decreases as the concentration increases. It is in equilibrium with its ions in water and its conjugate CH 3 COO a weak base is. Acetic acid is an example.
Which of the following statements is true of weak acids dissolved in water. Acid has a bitter taste. Phosphoric acid is a weak acid and is only partially dissociated into H and other ions some of which are also weak acids.
They are totally unionized in water. A strong base ionizes completely while a weak acid only ionizes partially. Acetic acid is a weak acid and only ionizes about 1 into hydronium ions which makes less acidic higher pH.
Therefore the nature of CH 3 COO is basic and we call CH 3 COO the conjugate base of CH 3 COOH. The electrical current generated in the solution is produced by. Hydrogen chloride HCl ionizes completely into hydrogen ions and chloride ions in water.
So when we add more and more of a solute into a solvent then there will occur an increase in the concentration of solute. NaOHs Naaq OHaq Substances that ionize completely which include strong acids and bases are called strong electrolytes and further include basically all water-soluble ionic compounds. The hydronium ions concentration increases.
Because HCl is a strong acid its conjugate base Cl is extremely weak. Hydrocyanic acid HCN ionizes partially in water according to the following equation. An acid has a bitter taste.
A weak acid is a weak electrolyte and weak electrolytes do not completely dissociate or ionize completely in water but are partially ionized or dissociate incompletely in water. It only partially ionizes in water. An acid or bases strength refers to its degree of ionization.
Since a solution consists of solute and solvent. Strong acids ionize completely when dissolved in water. The metal forms anions.
The following molecule is an example of which. They are completely ionized in water. A weak acid ionizes only to a small extent in water.
Carbon dioxide gas is produced. Because of this a. Hence it only ionizes partially into a solution.
A weak acid has a very low concentration.

Chapter 9 Acids Bases Ppt Download
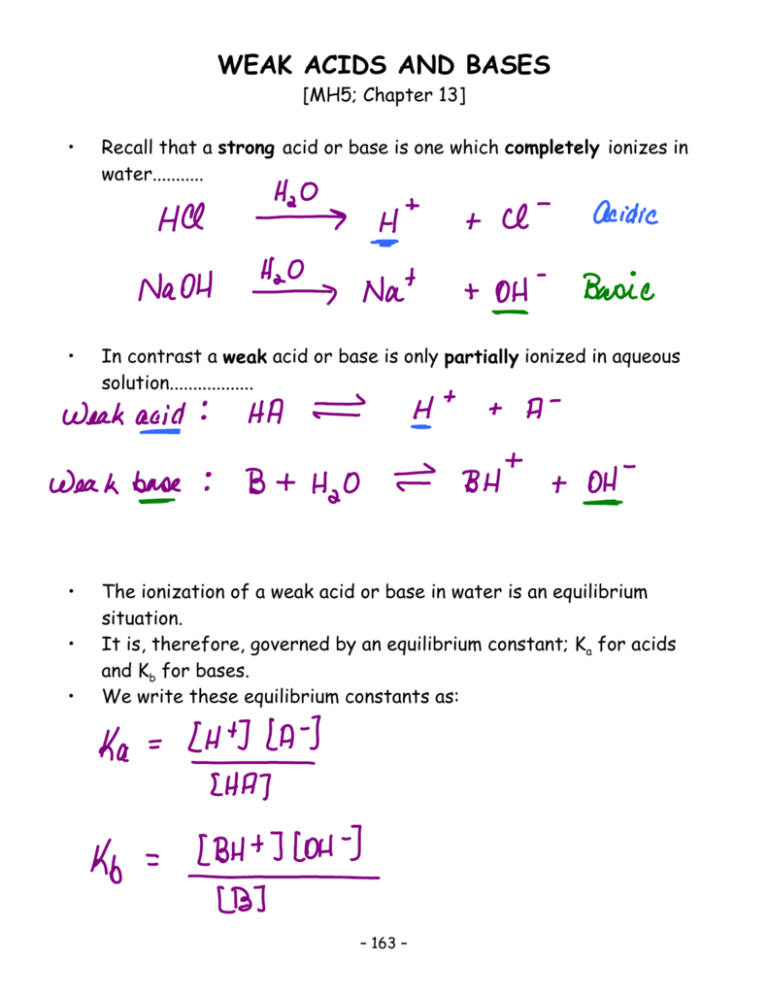
Completed Notes For Weak Acids And Bases

Ionization Of Acids And Bases Ionization Of Compounds Solved Examples
No comments for "Which of the Following Acids Ionizes Only Partially in Water"
Post a Comment